Fda Guidance 3D Printing Medical Devices. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. Food and drug administration released finalized guidance regarding 3d printing in medical devices. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. Other uses of 3d printing. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. The faster these approvals happen, the more the medical field can advance. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. List of fda tracked dental 3d printer resins.
Fda Guidance 3D Printing Medical Devices . Today 3D Printing Is Used In A Wide Variety Of Applications, Even For The Manufacture Of Medical Devices.
Use Of 3d Printing In The Manufacturing Of Medical Devices Emma International. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. Other uses of 3d printing. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. List of fda tracked dental 3d printer resins. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Food and drug administration released finalized guidance regarding 3d printing in medical devices. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; The faster these approvals happen, the more the medical field can advance. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology.
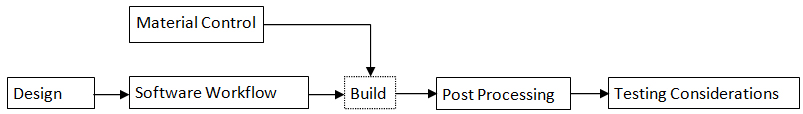
The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved.
In the past several months it has undergone revisions on the advice of medical. The medical device manufacturer should have objective evidence available that validation of the processing procedures has been undertaken to. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. Formlabs recommends you consult each of these sources before using any 3d printing technology to produce medical equipment. Mpo & odt editor sean fenske speaks with mike drues, president of vascular sciences, on recently finalized regulatory guidance for additive manufacturing and 3d printing of medical devices. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. List of fda tracked dental 3d printer resins. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. Today 3d printing is used in a wide variety of applications, even for the manufacture of medical devices. Food and drug administration (fda) published useful general guidance on the technical considerations for additive manufactured devices. The food and drug administration (fda) is responsible for the approval of medical devices in the united states of america. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Regulators issued the document on may 10 and are soliciting comments and. Other uses of 3d printing. In light of such changes, the fda issued its guidance for the 3d printing of medical devices in 2017. To receive device approval, manufacturers must prove that the device is equivalent to a product already. In 2017, the fda published specific guidance on 3d printing in technical considerations for additive manufactured medical devices. So what are fda's current thoughts regarding the approval of 3d. Food and drug administration released finalized guidance regarding 3d printing in medical devices. Medical device manufacturers can benefit from 3d printing in a number of different ways. Medical 3d printing is now an indispensable part of medicine. In the past several months it has undergone revisions on the advice of medical. (3dprintingchannel) the fda currently assesses 3d printed medical devices and conventionally made products under the same guidelines, despite the different manufacturing methods involved. Let's have a look at the most intressting applications of 3d printing in medicine and healthcare. The faster these approvals happen, the more the medical field can advance. 3d printing of tissues and organs. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Manufacturers should, however, be aware of the current downsides of 3d. Health canada published the first draft of its 3d printed medical device guidance in november 2018.
Use Of 3d Printing In The Manufacturing Of Medical Devices Emma International . Food And Drug Administration Released Finalized Guidance Regarding 3D Printing In Medical Devices.
Stratasys Medical Regulatory Information Stratasys. Other uses of 3d printing. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. List of fda tracked dental 3d printer resins. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Food and drug administration released finalized guidance regarding 3d printing in medical devices. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. The faster these approvals happen, the more the medical field can advance. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved.
How Fda Regulates 3d Printed Medical Devices Emma International : The Food And Drug Administration (Fda) Is Responsible For The Approval Of Medical Devices In The United States Of America.
3d Printing Meets Medical Devices Fda Weighs In On Additive Manufacturing Morrison Foerster Llp Class Dismissed Jdsupra. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Food and drug administration released finalized guidance regarding 3d printing in medical devices. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. The faster these approvals happen, the more the medical field can advance. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos.
Stratasys Medical Regulatory Information Stratasys , Manufacturers should, however, be aware of the current downsides of 3d.
Insights Into The New Fda 3d Printing Guidance How It Impacts Med Device New Product Development. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Food and drug administration released finalized guidance regarding 3d printing in medical devices. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. The faster these approvals happen, the more the medical field can advance. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. List of fda tracked dental 3d printer resins. Other uses of 3d printing.
Fda Announces 3d Printed Medical Device Guidance Covering The Specialized Field Of Orthopedic Product Development And Manufacturing . Formlabs Recommends You Consult Each Of These Sources Before Using Any 3D Printing Technology To Produce Medical Equipment.
Medical Applications Of 3d Printing Fda. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Food and drug administration released finalized guidance regarding 3d printing in medical devices. Other uses of 3d printing. The faster these approvals happen, the more the medical field can advance. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. List of fda tracked dental 3d printer resins. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance.
Covid 19 Digital Manufacturing And 3d Printing Response 3d Systems , Medical 3D Printing Is Now An Indispensable Part Of Medicine.
How The Fda Is Working Towards Approving 3d Printed Medical Devices Grabcad Blog. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; Other uses of 3d printing. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. Food and drug administration released finalized guidance regarding 3d printing in medical devices. The faster these approvals happen, the more the medical field can advance. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. List of fda tracked dental 3d printer resins.
Meeting New Fda Quality Management Requirements For Additive Manufacturing Software 3dprint Com The Voice Of 3d Printing Additive Manufacturing : 3D Printing Of Tissues And Organs.
3d Printed Medical Devices Given Fda Draft Guidance Medilink West Midlands. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Other uses of 3d printing. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; Food and drug administration released finalized guidance regarding 3d printing in medical devices. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. The faster these approvals happen, the more the medical field can advance. List of fda tracked dental 3d printer resins. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical.
Fda Issues Guidance For 3d Printed Medical Devices Digital Engineering 24 7 - The Fda Guidance Encompasses Technical Design And Manufacturing Considerations And Device Testing Considerations, Which Deal Respectively With Quality System Compliance And Premarket Review Submissions.
3d Printing Medical Devices Market Size Industry Analysis Forecast. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. Other uses of 3d printing. Food and drug administration released finalized guidance regarding 3d printing in medical devices. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. The faster these approvals happen, the more the medical field can advance. List of fda tracked dental 3d printer resins. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area.
Meeting New Fda Quality Management Requirements For Additive Manufacturing Software 3dprint Com The Voice Of 3d Printing Additive Manufacturing , So What Are Fda's Current Thoughts Regarding The Approval Of 3D.
Fda Provides First Ever Official Guidance For 3d Printing Of Medical Devices 3d Printing Media Network The Pulse Of The Am Industry. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. Other uses of 3d printing. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Food and drug administration released finalized guidance regarding 3d printing in medical devices. List of fda tracked dental 3d printer resins. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. The faster these approvals happen, the more the medical field can advance.
3d Printing In Healthcare Where Are We In 2019 Amfg : In Light Of Such Changes, The Fda Issued Its Guidance For The 3D Printing Of Medical Devices In 2017.
3d Printing Of Medical Equipment Can Help In The Pandemic But Is Only A Stopgap The Pew Charitable Trusts. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology. The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. The faster these approvals happen, the more the medical field can advance. Food and drug administration released finalized guidance regarding 3d printing in medical devices. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; List of fda tracked dental 3d printer resins. Other uses of 3d printing. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing.
Fda Provides First Ever Official Guidance For 3d Printing Of Medical Devices 3d Printing Media Network The Pulse Of The Am Industry . Fda Describes Guidance Document As Leapfrog Guidance Because It Provides Agency's Initial Thoughts On This Emerging Technology.
Frontiers Progressive 3d Printing Technology And Its Application In Medical Materials Pharmacology. The faster these approvals happen, the more the medical field can advance. Devices manufactured by 3d printing are generally subject to the same regulatory requirements as more traditionally manufactured medical. Food and drug administration released finalized guidance regarding 3d printing in medical devices. List of fda tracked dental 3d printer resins. 3d printing (also known as additive manufacturing) is one of the more exciting recent developments in the medical device area. Therefore they are evaluated according to the for further information on custom device exemptions, please refer to the custom device exemptions guidance. The fda classifies medical devices into three categories based on the level of control necessary to ensure the device's safety and effectiveness. Medical device manufacturers should refer to fda guidance documents and quality systems regulations for more information on specific fda's thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3d printing. The fda regulates 3d printed medical devices through the same pathways as traditional medical devices; The new guidance outlined for the consideration of new 3d printed medical devices sets up parameters and regulations for manufacturers who want to get their 3d printed products fda approved. The fda guidance encompasses technical design and manufacturing considerations and device testing considerations, which deal respectively with quality system compliance and premarket review submissions. Each device or type of device has its own set of tests that may be based on fda guidance documents, international standards, or internal process controls. Other uses of 3d printing. 3d printing industry asked some of the enterprises leading this new era for their reaction to the fda's guidance for manufacturers submitting 3d printed devices laura gilmour, global medical business development manager at eos. Fda describes guidance document as leapfrog guidance because it provides agency's initial thoughts on this emerging technology.


